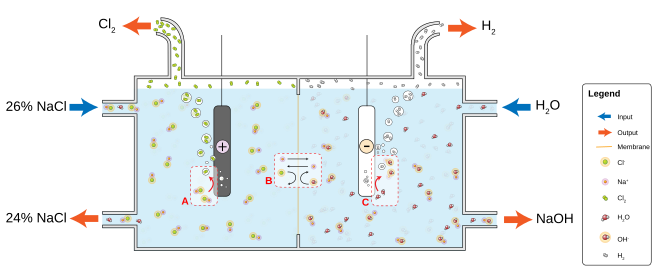
The production of chlorine caustic soda and hydrogen is obtained by electrolysis of a concentrated nacl solution. In the mannheim process and in the hargreaves process sodium chloride is used for the production of sodium sulfate and hydrochloric acid.
Production Of Pharmaceutical Grade Sodium Chloride From Rock Salt Using Download Scientific Diagram
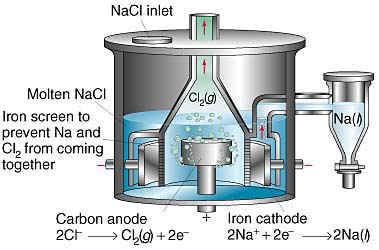
The products of the electrolysis of molten fused sodium chloride are sodium metal and chlorine gas.

Production of sodium chloride. Contract and relax muscles. Sodium chloride has an international standard that is created by astm international. The standard is named astm e534 13 and is the standard test methods for chemical analysis of sodium chloride.
Its color is intensely greenish yellow. The second largest consuming segment 15 in 2019 is road salt for deicing. It s made when na sodium and cl chloride come together to form white crystalline.
Most of the artificial brines are obtained by pumping water into underground salt beds. Chlorine dioxide provides the source of chlorine that is converted to sodium chlorite. The following pie chart shows world consumption of sodium chloride.
Consumption for deicing can vary substantially from one year to another depending on climatic conditions. The primary raw materials used in the production of sodium chlorite are chlorine dioxide sodium hydroxide and hydrogen peroxide. Sodium chloride is commonly called rock salt table salt.
These methods listed provide procedures for analyzing sodium chloride to determine whether it is suitable for its intended use and application. Salt is an inorganic compound meaning it doesn t come from living matter. Chlorine gas bubbles out of the melt above the anode.
Commercial salt is manufactured from rock salt as well as from seawater and other natural and artificial brines. Weak or depleted dechlorinated brine from the membrane cells is returned to saturation by dissolving additional nacl salt in the brine saturation unit. Salt is mostly produced by evaporation of seawater.
Chlorine dioxide is a gas at room temperature. Chemical production accounts for over half of all salt use globally. Liquid sodium floats to the top of the melt above the cathode and is drained off into a storage tank.
Electrolysis cell for molten sodium chloride a commercial electrolysis cell for the production of metallic sodium and chlorine gas from molten nacl. In the downs cell a circular iron cathode surrounds the carbon anode and the products of the electrolysis are separated by steel mesh or gauze to prevent them coming into contact and reforming sodium chloride.
 Measurement Of Concentration Of Supersaturated Nacl Solution In Salt Dissolvers At Electrolysis Plants Pt Yokogawa Indonesia
Measurement Of Concentration Of Supersaturated Nacl Solution In Salt Dissolvers At Electrolysis Plants Pt Yokogawa Indonesia
 Electrolysis Of Sodium Chloride Introduction To Chemistry
Electrolysis Of Sodium Chloride Introduction To Chemistry
 Sodium Chloride Chemical Economics Handbook Ceh Ihs Markit
Sodium Chloride Chemical Economics Handbook Ceh Ihs Markit
Gcse Chemistry Year 10 Preparation Of Crystals Of Sodium Chloride Experimental Sheet
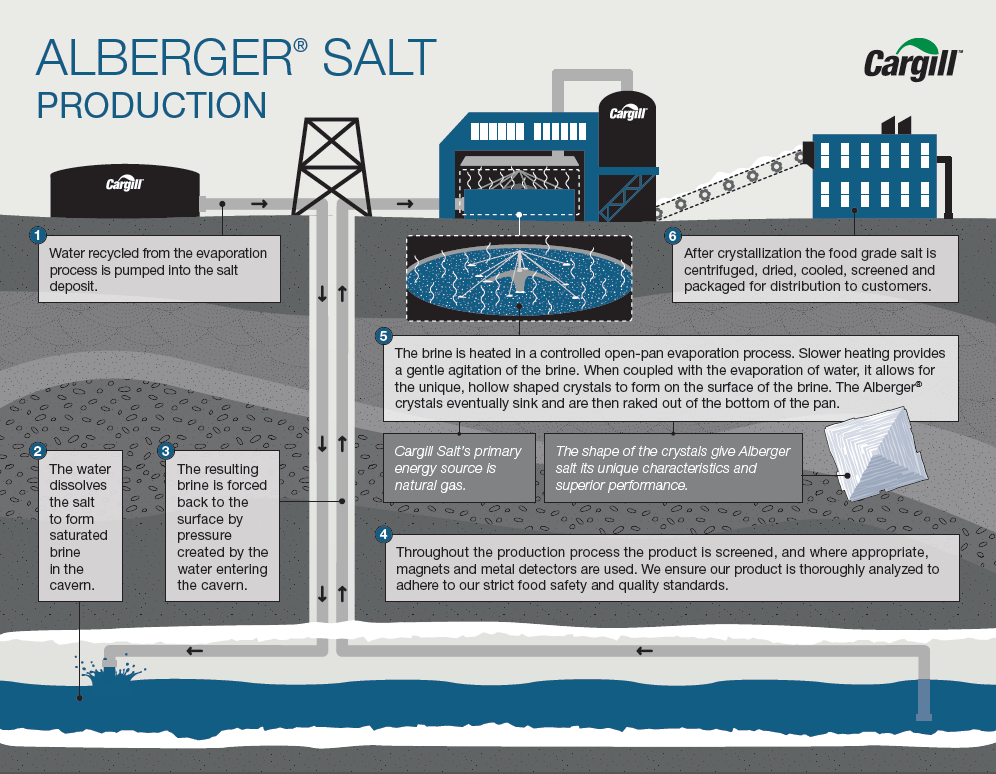 Cargill Salt Group S Salt Manufacturing Processes Cargill
Cargill Salt Group S Salt Manufacturing Processes Cargill
 Combined Production Of Nacl And Na So Sep Salt Evaporation Plants Ltd
Combined Production Of Nacl And Na So Sep Salt Evaporation Plants Ltd
Igcse Chemistry 5 29 Describe The Manufacture Of Sodium Hydroxide And Chlorine By The Electrolysis Of Concentrated Sodium Chloride Solution Brine In A Diaphragm Cell
How Salt Is Made Material Used Processing Procedure Industry Machine Raw Materials
.jpg)