Place the orthosilicic acid into a heat safe glass or porcelain dish and heat it over a burner flame for about 5 minutes. Sand is non toxic but it presents an inhalation hazard since the small particles could become trapped in your lungs if inhaled.
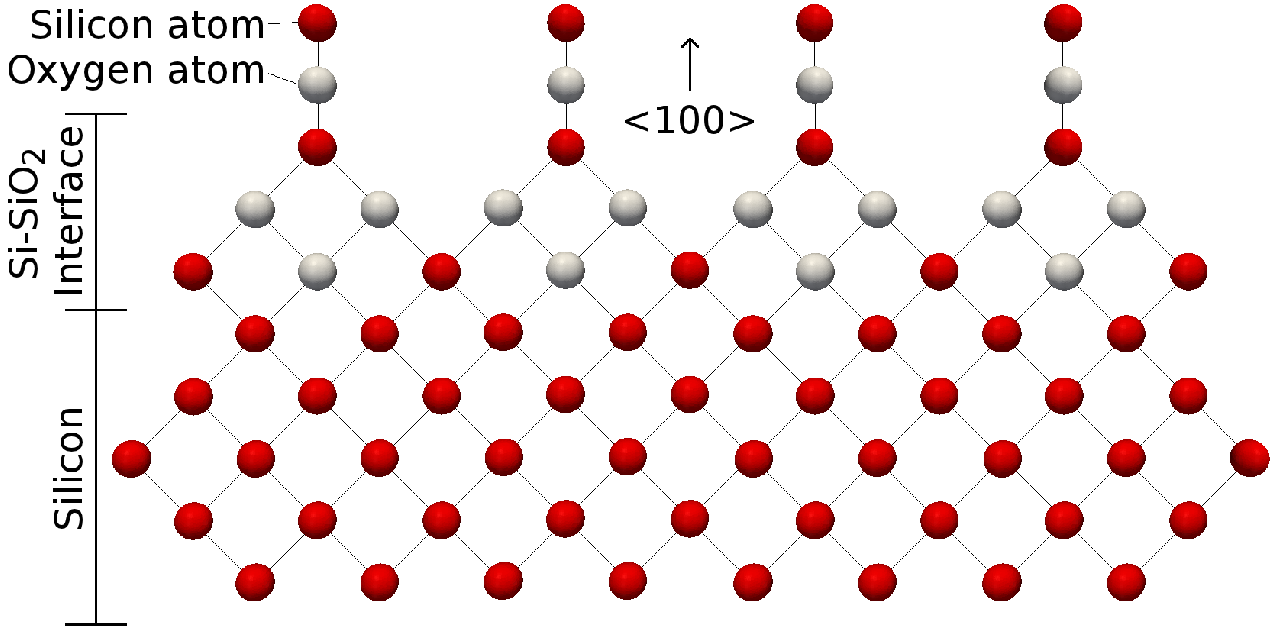 2 1 1 Molecular Structure Of The Silicon Silicon Dioxide Interface
2 1 1 Molecular Structure Of The Silicon Silicon Dioxide Interface
Tetraetyl orthosilicate teos is usually used as precursor to synthesize silicate colloids by undergoing hydrolysis or gelation and condensation or polymerization process chrusciel and slusarki 2003.
How to make silicon dioxide. The orthosilicic acid dries to form silicon dioxide sio 2 which is your pure sand. Its gel is washed and dehydrated to make colourless microporous silica. The reaction involving a trisilicate along with sulfuric acid is provided below.
Organic compounds containing silicon dioxide undergo chemical reaction to form silicon dioxide sio 2 by the sol gel process. Production of silicon dioxide the acidification sodium silicate solutions obtain precipitated silica or amorphous silica.