The chemical structure super glue is a very specific type of glue made of the ingredient cyanoacrylate. This family includes methyl 2 cyanoacrylate super glue ethyl 2 cyanoacrylate krazy glue n butyl cyanoacrylate and 2 octyl cyanoacrylate used in medical applications etc.
 Chapter 29 6 Solutions Organic Chemistry 8th Edition Chegg Com
Chapter 29 6 Solutions Organic Chemistry 8th Edition Chegg Com
Applying super glue cyanoacrylate to cotton or wool results in a rapid chemical reaction that releases enough heat to cause minor burns so typically this should be avoided.

Super glue chemical reaction. However if enough cyanoacrylate is added to the cotton or wool the fabric will. Superglue is now a 400 million industry and is now used in many sectors of the chemical and engineering industries. Under magnification it is possible to see how the ca forms microscopic chains as the molecules attach to one another.
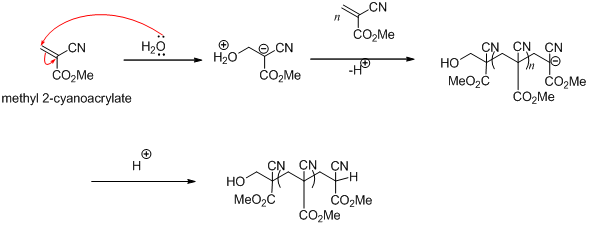
The reaction with water is also the reason that getting superglue onto your skin can easily end badly. Their general chemical formula is ch 2 c cn co 2 r with r representing an organic e g methyl ch 3 molecular group. Cyanoacrylates are a family of strong fast acting adhesives.
This is the condensation of formaldehyde methanal and an alkyl cyanoacetate. Super glue chemically reacts with cotton or wool to generate enough heat to start a fire. Ca cyanoacrylate monomer super glue polymerization a chemical reaction causing molecules of ca to rapidly bond to one another.
Just keep it away from your favourite cotton jumper and sheep 1 whilst the majority of molecules in a glass of pure water are h2o a small amount can react together to form positively charged h3o ions this is essentially what makes acids acids and negatively charged oh ions. Super glue is great and its use in medicine shows it to be so much more than just a super tool for diy. There s a very good reason why superglue packets warn you not to get it anywhere near your eyes or mouth.
As your skin contains moisture it too can set off the polymerisation reaction. Therefore the most common trigger for superglue is hydroxyl ions oh in water which is convenient since almost any object you might wish to glue will have at least some traces of water on its surface. Polymerization retardants are added to ca to slow this reaction.
This allows for a unique feature to super glue that is moisture alone can start polymerization. The synthesis of cyanoacrylate is based on the knovenagel reaction 2. Owing to the highly polar nature of the nitrile cn and ester rcoor groups these compounds react quickly to any basic surface especially in the presence of moisture.